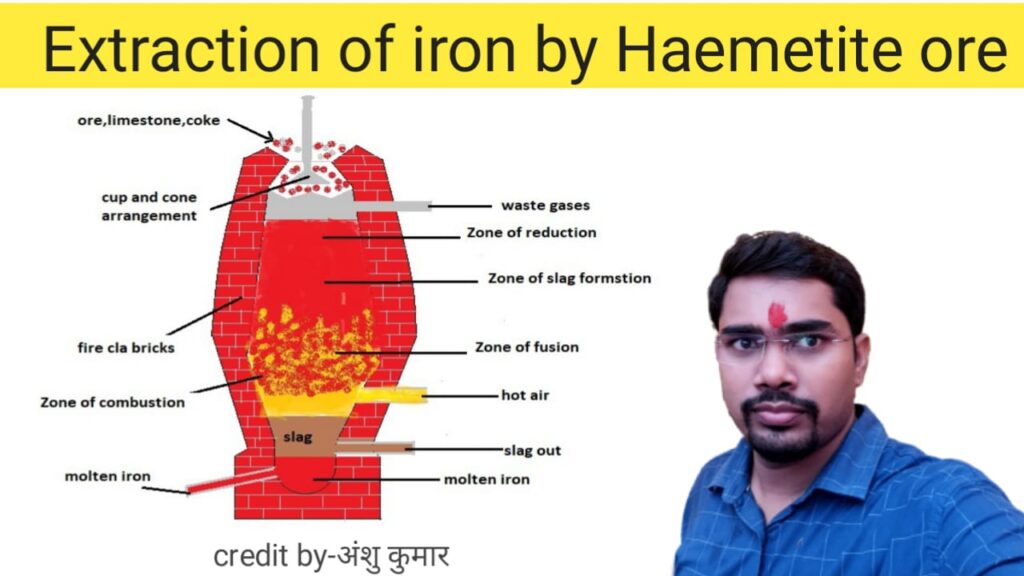
Catalytic property and formation of industrial compounds in transition elements
Catalytic Property :- Transition element shows catalytic property due to following reason (a) Such elements are in solid state so reaction is taken on its surface. (b) Tendency to show variable oxidation state. (c) Presence of empty d-orbital. (d) They have small size. Ex:- Pd,Pt,Ni,Co Mn V are the most popular transition metal with shows …
Catalytic property and formation of industrial compounds in transition elements Read More »