Extraction of iron by haematite ore(Fe2O3).
Extraction of Iron :- Iron is extracted by haemetite are which consist impurity of silica , phosphorus , carbon etc. Extraction of it completed in following steps :-
Concentration of Ore :- Such ore is concentration by gravity separation or Hydraulic washing.
Calcination :- Concentrated is strongly heated in limited supply of oxygen then volatile impurity removed as well as impurity removed of non-metal also removed in the form of their oxide.
Fe2O3 . 2H2O Fe2O3
Fe2O3
4P + 5O2 → 2P2O5
4As + 3O2 → 2As2O3
Carbon reduction process :- Reduction of iron oxide by carbon in presence of strong heat is called smelting which occours in blast furnace. Blast furnace :- It is long tower like structure which is conical at the top and border in the middle and again narrow at the bottom. It is made up of fire proof pricks and the inner wall of it is lined by silica. At the bottom of the furnace blast of hot air passed inside furnace. In such furnace these are four different zones which has different temperature and in which has different chemical reactions are taken.
- Zone of combustion
- Zone of fusion
- Zone of slag formation
- Zone of reduction
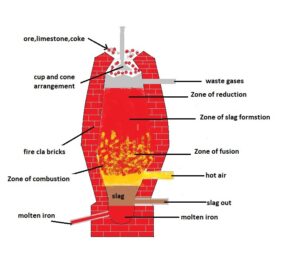
- Zone of combustion :- It is present at the lowest part of the furnace. The temperature of such zone is 2200k. From the bottom of the furnace blast of hot air passed inside it which react with upcoming coke such reaction is highly exothermic and temperature of such zone become 2200k.
C+ O2 → Co2 + Heat
Zone of fusion :- Such zone is present above zone of combustion. Co2 formed in zone of combustion rises up and react with upcoming coke and form CO. Such rkn is endothermic in nature so temperature of such zone is decreased and reached upto 1570k.
CO2 + C → 2CO – Heat
In such zone reduced iron coming from zone of reduction get fused in such zone and also some of the from oxide also get reduced into iron.
Fe2O3 + 3CO → 2Fe + 3CO2
Fe(s) → Fe (l)
Zone of slag formation :- In such zone limestone get dissociated and form calcium oxide along with Co2. This CaO act as flux which react with impurity of silica and form slag.
CaCO3  CaO + CO2
CaO + CO2
CaO + SiO2 → CaSiO3 (slag)
(Impurities) (Flux)
Slag form in such zone covered the molten iron and prevent it further oxidation of molten iron.
Zone of reduction :- It is upper zone of the furnace here temperature is lowest i.e 875k with respect to other zone. In such zone iron oxide reduces by CO into another form of iron oxide which further reduces by coke into iron.
Fe2 + CO  2FeO + CO2
2FeO + CO2
FeO + C Fe + CO
Fe + CO
From the bottom of the furnace lets collect molten iron and slag in different container. This molten iron is called Pig iron.
It steel consist impurity more than 5%. This pig iron change into cast iron by heating of it with scrape of iron in presence of blast of hot air. After that it becomes cast iron which is more than pig iron.
Formation of wrought Iron :-
Such iron is the purest form of iron. It consist impurity up to only 0.5%. When cast iron is heated in Reveroratery furnace along with haematite are then wrought iron is obtained. Impurity present in cast iron get oxidises by haematite are which provide oxygen for the oxidation of such impurity.
2Fe2O3 + 3C → 4Fe + 3CO2
2Fe2O3 + 3S → 4Fe + 3SO2
Fe2O3 + 3Mn → 2Fe + 3MnO
MnO + SiO2 → MnSiO3 (slag)
Extraction of iron,FAQ.
Q.What is the concentration of iron in hematite?
Ans-Concentration of iron by gravity separation or Hydraulic washing.
Q.What is the ore hematite ore?
Ans- Hematite ore of iron.
Q.Which metals ore is hematite?
Ans- Iron
Q. Formula of hematite is…..?
Ans-Fe2O3
Q. Most popular ore of iron is……?
Ans-hematite.
Q. During extraction of iron in which type of slag is obtained.
Ans- CalciumSilicate(CaSiO3)