Adsorption Isobar
Knowing the effect of temperature on adsorption we plot a graph between rate of
adsorption and temperature at constant Pressure is called adsorption isobar.
Case-1 In chemical adsorption initially rate of adsorption increase by increase in
Temperature after that it decreases by increase in temperature.
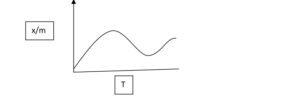
Case-2 In physical adsorption rate of adsorption decreases by increase in temperature
Because energy of adsorbed increase so it desorbed.
Application of adsorption
- It helps in purification in sugar by using activated charcoal.
- It helps in purification of air.
- Such technique is used in chromatography, it is a technique to separate Impurities by using proper adsorbent.
- Such technique is used in paint industry,spirit is used as adsorbent through adsorb gaseous impurities present in paint.
- Adsorption is used in catalysis.
Catalyst
It is the substance which change the rate of reaction without participated in chemical
Reaction is called catalyst.
Eg:- Iron, Aluminium, Nickel…..etc are act as catalyst in various chemical.
Types of catalyst :- It is two types.
(1) Homogeneous catalyst
Those catalyst which are in same phase or physical state in which reactant and products are present.
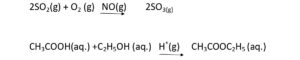
(2) Hetrogeneous catalyst
Catalyst having different physical state with respect to reactant and products.
